pfizer alopecia drug
PF-06700841 is a tyrosine kinase TYK 2JAK1 inhibitor. Whenever the hair is absolutely tumbling from the head it is named Alopecia Totalis then again if going uncovered is seen all through the entire body it is called Alopecia Universalis.
 |
| Ridiculous Viral Oscars Theory Says Pfizer Staged Slap To Promote Alopecia Drug |
The drug Etrasimod is a once-a-day tablet being trialled to.
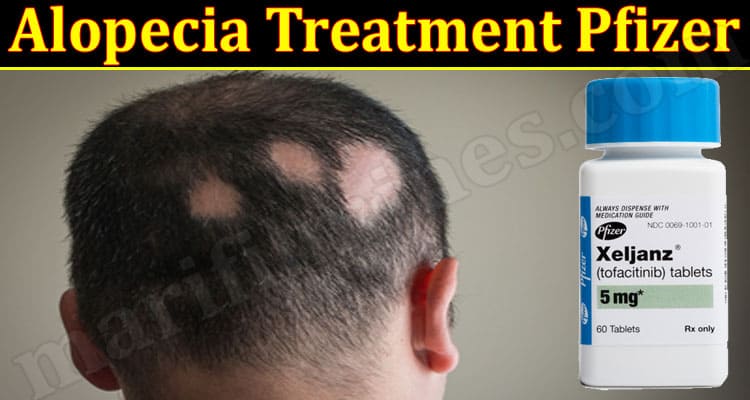
. Alopecia areata is an autoimmune disease resulting in hair loss which can also affect fingernails and toenails. Global pharmaceutical giant Pfizer Inc. An increased proportion of trial subjects receiving ritlecitinib 50mg or 30mg for 24 weeks had scalp hair loss of 20 or less. Hair loss following SARS-CoV-2 vaccination is an increasingly reported phenomenon in.
Pfizer has started a phase 2b3 clinical trial of PF-06651600 an oral JAK3 inhibitor to treat patients with moderate to severe alopecia areata. Pfizer is currently in phase-three clinical trials of their new drug Etrasimod which is developed to provide long-term treatment for various. 50 mg oral tablets. Has announced that its current trial on a potential treatment for alopecia areata achieved its primary efficacy endpoint.
Conclusion This article portrayed Pfizer Alopecia 2022 Drug information and found that Etrasimod is the medicine Pfizer suggested for Alopecia Areata. Pfizer was indeed one of the Oscars co-sponsors and one of its subsidiaries Arena is developing a treatment called etrasimod spelled all. NEW YORK--BUSINESS WIRE--Pfizer Inc. 12 Ritlecitinib 50 mg and 30 mg.
Pfizers ritlecitinib joined Lilly and Incytes Olumiant on the list of drugs to hit the primary endpoint in pivotal alopecia trials and Concert Pharmaceuticals is following closely behind. But neither drug will be available to the public in the near future. Pfizer world headquarters in Manhattan New York. Pfizer is currently developing a drug to treat alopecia and other immuno-inflammatory diseases.
PF-06651600 is an oral Janus kinase JAK 3 inhibitor. Pfizer said Wednesday an experimental drug central to its 7 billion purchase of Arena Pharmaceuticals succeeded in a crucial Phase 3 trial in patients with moderate to severe ulcerative colitis. Pfizer has reported positive top-line data from the Phase IIbIII ALLEGRO clinical trial of its drug ritlecitinib in alopecia areata patients. Jenna Jameson L has floated the theory that Will Smith R slapping Chris Rock at Sundays Oscars was orchestrated by Pfizer to push its alopecia drug which the company reported positive tiral.
Pfizer has two medications ritlecitinib and etrasimod under development to treat alopecia areata. In addition to ulcerative colitis Pfizer plans on studying etrasimod in atopic dermatitis Crohns disease a type of esophageal inflammation and in alopecia areata. Pfizers ritlecitinib joined Lilly and Incytes Olumiant on the list of drugs to hit the primary endpoint in pivotal alopecia trials and Concert Pharmaceuticals is following. Pfizer announced results from its Phase IIa clinical trial of PF-06651600 and PF-06700841 in alopecia areata AA.
There are currently no FDA-approved treatments for alopecia an autoimmune disease characterized by hair loss but multiple drugs could come to market in the indication over the coming years. It is possible that the messenger RNA SARS-CoV-2 Moderna and Pfizer vaccines can trigger a T cell-mediated immune response with the downstream effects of alopecia. The study dubbed Elevate 12 found that patients given the former Arena drug etrasimod had meaningfully higher remission rates after 12 weeks than those who received a. Pfizers ritlecitinib meets primary efficacy goal in alopecia areata trial.
It is more likely that Pfizer was sponsoring the event to promote their COVID vaccine but Ill admit that it is curious that now the entire world is talking about alopecia as this race for a. Should development succeed etrasimod could potentially blunt the impact of Xeljanz losing patent protection which Pfizer expects to occur in 2025. In a statement Pfizer said that the Phase IIbIII ALLEGRO trial on the oral once-daily ritlecitinib drug was found to be effective against the autoimmune disease which is characterized by an immune attack on. NEW YORK--BUSINESS WIRE-- Pfizer Inc.
PFE today announced positive top-line results from the Phase 2b3 ALLEGRO trial evaluating oral once-daily ritlecitinib in patients with alopecia areata an autoimmune disease driven by an immune attack on the hair follicles that causes hair loss on the scalp and can also affect the face and body. NYSEPFE today announced its investigational oral Janus kinase 3 JAK3 inhibitor PF-06651600 received Breakthrough Therapy designation from the US. Pfizer top Oscars sponsor at work on drug for the auto-immune disorder alopecia April 6 2022 More than 40000 Wisconsin voters in. Patients participating in the vaccine sub-study will receive the 2 vaccines or one of the 2 vaccines at the vaccine sub-study Day 1 which will occur at a scheduled study visit on or after the Month 9 visit and prior to or on the Month 32 visit of the main B7981032 study.
Food and Drug Administration FDA for the treatment of patients with alopecia areata a chronic autoimmune skin disease that causes hair loss on the scalp face or body12.
 |
| Alopecia Treatment Pfizer March Useful Information |
 |
| Did Pfizer Plan Oscar Slap To Promote Alopecia Drug A Factcheck |
 |
| Pfizer Alopecia Drug 2022 April How Related To Oscar |
 |
| Pfizer Alopecia 2022 Drug March Read All About It |
 |
| Pfizer Evaluates Safety Of New Jak Inhibitor Alopecia Areata Drugs |
Posting Komentar untuk "pfizer alopecia drug"